Shop Electrodes by Application
Our products can be used in a wide range of applications. Our electrodes are manufactured accurately with first-class engineering and are ideal for quality results in various applications.
A pH probe is a device that measures the pH level of a substance or solution. It does this by measuring the amount of hydrogen (H+) or hydroxyl (OH-) ions in a substance. If there are more H+ than OH- ions in a solution, then the acidity goes up. On the other hand, alkaline solutions will have more OH- ions than H+ ions. We represent the acidity or alkalinity of a substance on a pH scale. A pH level of 0 is highly acidic, 14 is highly alkaline, and 7 is neutral.
Measuring the pH level of a substance is crucial in many industries including food production, cosmetics, and pharmaceuticals. So, to answer the question, “what is a pH probe?”, we need to look at its components, how it works, and what industries use it.
When looking at the question, “what is a pH probe?”, there are several main components of the structure to look at:
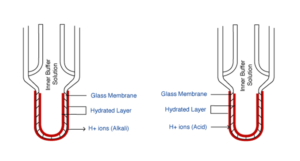
An electrode requires two ‘cells’ in order to complete an electrical circuit and give a reading from a pH meter. These are the pH cell and the reference cell, and in most electrodes they are both within the same casing to form a combination electrode. When the two cells are used in the form of two separate electrodes, they are called half cells. The pH cell is made up of a glass membrane, which is specially formulated to be sensitive to hydrogen ions. When the glass membrane comes into contact with an aqueous sample, a hydrated layer forms on the inside and outside of the membrane. Hydrogen ions will transfer across this membrane, and the direction of the transfer depends on whether the sample is acidic or alkaline. The difference between the inner and outer charge gives a membrane potential which can be compared to the stable reference potential to measure the pH value. The reference cell is stable as it is in contact with the sample through a junction rather than a hydrogen sensitive membrane. A meter calculates the difference in the pH and reference cell potential and gives a value in millivolts or is converted to pH units.
The composition of most pH electrodes will consist of a pH sensitive membrane fused to a glass stem. The stem is filled with a neutral conductive electrolyte in which a silver/silver chloride wire is placed to form an electrical connection. The reference consists of a silver/ silver chloride wire encased in a potassium chloride electrolyte, and a junction. The junction is the communication channel between the reference cell and the sample, and can be made out of different materials depending on the application and type of sample.
Many industries need to use pH probes so that they can measure the quality of their products. The food and beverage industry needs to measure the quality of dairy, alcohol and meat products to avoid the risk of selling spoiled, unsafe, or poor tasting products. When it comes to construction, measuring the pH level of metals helps find ways to prevent materials from wearing down or corroding. Likewise, measuring pH levels in wastewater treatment helps ensure the water does not harm equipment or the environment. pH levels also need to be measured in swimming pools and Jacuzzis to reduce the risk of eye and skin irritation, corrosion, and ensure that chlorine in the water is effective.
Here at Sentek we are a leading UK manufacturer of glass pH probes for all industries. To find out more about our range of pH electrodes, please browse our range online or speak with our expert team today.
Copyright 2024 Sentek Limited. All Rights Reserved.
Marketing by Unity Online