Shop Electrodes by Application
Our products can be used in a wide range of applications. Our electrodes are manufactured accurately with first-class engineering and are ideal for quality results in various applications.
In this article, we delve into the anatomy of pH electrodes. We will explore key elements; including the reference system, electrolytes, membrane, and the electrode body. Understanding the inner workings of a pH electrode is beneficial to achieve precise and accurate pH measurements for your specific application.
A pH probe is one example of many ion selective electrodes (ISE) that are produced at Sentek. ISE and pH measurement are used from lab applications to work in the field. Sentek manufactures probes for a variety of customers with applications including engineering projects, process control, agricultural, environmental, medical, pharmaceuticals and food and the brewing industry.
pH is a quantitative measurement of the acidity or basicity of an aqueous solution; this is directly related to the concentration of hydrogen ions (H+) or hydroxide ions (OH–) in the solution. When the concentration of OH– is equal to that of H+ the solution is considered neutral which occurs at pH 7. As the amount of H+ in the solution increases the pH of the solution decreases and as the OH– increases the pH increases.
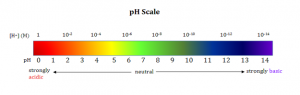
Figure 1 Showing an example pH scale and the logarithmic relationship between the pH scale and the H+ concentration. Source Microsoft Bing images
pH is a logarithmic scale defined by pH =-Log10 (aH+). This means that at pH 6 the activity of the hydrogen ion is ten times greater than that at pH 7, and pH 5 is 100 times greater than pH 7. A pH electrode uses potentiometric techniques to determine the activity of hydrogen ions in a given solution using a potentiostat. The measured potential describes hydrogen activity by the Nernst equation:
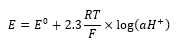
In order to measure the potential difference a pH probe requires not only an H+ sensitive membrane but also a non sensitive stable reference to compare against.
When looking at the anatomy of a pH electrode, the most common type of pH electrode in use today uses the silver/silver chloride (Ag/AgCl) reference electrode. In these cells a reversible redox reaction is occurring between solid AgCl and solid Ag and dissolved Cl–. A redox reaction describes how a reaction progresses via either the loss or gain of an electron.
Half-cell equation of Ag/AgCl:
![]()
The Ag/AgCl electrode will offer a stable and reproducible potential regardless of pH which makes it perfect for a reference electrode. The most common type of reference electrode used today is the silver/silver chloride due to its stable reading and non-toxic materials. Mercury/mercurous chloride (Hg/Hg2Cl2) is another type of reference electrode used; Calomel electrodes are less prone to blockages and are considered to be extremely stable, however due to the toxicity of mercury, it is generally avoided for the much safer silver/silver chloride (Ag/AgCl) reference.
Half-cell equation of Hg/Hg2Cl2:
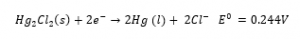
The half-cell electrochemical potentials(E0) above for AgCl and the Calomel only show half the picture. In order to measure the potential difference another electrode is required to measure against. The half cells in this scenario would have been measured against the standard hydrogen electrode (SHE):
![]()
The SHE is an arbitrary zero point to measure all other potentials against. Due to the impracticality of using hydrogen gas the SHE is only very rarely used. In a normal pH electrode, the other half cell is normally a silver/silver chloride electrode behind a H+ sensitive membrane.
Once the pH membrane and the reference electrode are in the test solution the circuit is complete, with the only varying potential being the working electrode inside the membrane glass. The change in H+ on the outside of the membrane glass will change the potential of the working electrode which can then be compared to the reference electrode. This is why reference electrodes have to be completely insensitive to a change in pH and have a stable potential.
The anatomy of pH electrodes can change for many reasons, one being that electrodes can come as a mono or part of a combination for convenience depending on the application. The reference is in contact with the sample solution via some type of porous material such as cotton, teflon and ceramic. Each of the junction types have variable characteristics depending on the requirements of the electrode.
Ceramics are a robust junction material that have low flow rates but high junction resistance. Ceramics have very stable readings this makes them a great general purpose junction material. Due to the small holes and low flow rate ceramic junctions are prone to blockages and need to be kept clean, this can be combatted by using multiple ceramic junctions.
Teflon junctions have a higher flow rate and are therefore less prone to blocking than a ceramic junction. Due to the lower maintenance of teflon junctions these are easier to handle.
The simplest type of junction would simply be a small hole called a ground joint, this would offer the lowest junction potential and the highest flow rate. These are simple to clean but will require a lot of re-filling, great for viscous samples or samples with lots of suspended solids.
A double junction or even a triple junction could extend the life of an electrode in harsh environments. An additional cavity filled with electrolyte and another junction is used to further separate the reference from the sample. Double junctions are often used when a sample is particularly hostile to the reference in use, however, the junction potential of all of the internal junctions used will have to be considered as well the external junction and the types of electrolyte(s) used. Using an electrolyte similar to the sample medium in the outer chambers can decrease the amount of overall junction potential.
Sentek has also developed an iodine/iodide pH and reference system which offers a fast response time and a low temperature sensitivity.
Figure 2 shows a diagram of Sentek electrode with labels pointing to all of the key components of a pH electrode
Potassium chloride (KCl) solution is the most common type of electrolyte used in pH probes, it allows for a connection i.e. a salt bridge between the reference electrode and the membrane glass. When reviewing the anatomy of pH electrodes for your experiments, consider that different types of electrolytes are available for different applications, which can be key for decreasing the junction potential for more precise measurements. An advantage of KCl is that the diffusion rates of the anion and cation are very similar which helps to establish a stable junction potential. The electrolyte can be made into varying types of solutions and gels to fit any requirements. The combination of junction material and electrolyte will determine the flow of the electrode. Standard KCl solution is a free-flowing liquid that will pass quickly through a junction, this will keep the junction wetted through and allow for good connection from the electrode to the sample, this type of electrolyte is well suited to the controlled lab environment where high precision is key. Gelled electrolytes are used more in the field, these restrict the flow of the electrolyte whilst still allowing for a good connection from the electrode to the sample solution, having a thicker gel will not only last longer between re-fills but will also help protect the reference from contamination, these are well suited to work in the field and industrial applications. Sentek has developed pH electrodes and reference electrodes that enable pH measurement in a variety of difficult samples e.g. non-aqueous samples, soil samples, high pH, high salt concentrations as well as slurries and viscous samples.
For a pH probe to make a potentiometric measurement two electrodes are required, one stable pH insensitive electrode and another behind an ion selective membrane. In this case our target ion is H+ and the membrane glass provides us with our selectivity. All membrane glass is specially made to be a good ionic conductor to allow the transfer of charge from the outside of the pH bulb to the inside. As a pH bulb is immersed in an aqueous solution the outer layers of Si-O groups become protonated by H+, this is the formation of what is known as the leached layer. The ionic equilibrium shown below is the key element in the selectivity of the membrane:
![]()
There are two glass membrane/solution interfaces, one outside of the membrane and one on the inside of opposing polarity. The internal solution is stable as it is completely sealed so the difference in potential arises from the internal glass membrane/solution interface. The difference of the inner glass leached layer and the outer glass leached layer.
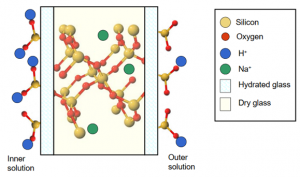
Figure 3 A diagram showing a not to scale illustration of how the charge is transferred via alkali metals to the inner solution from outside of the electrode. pH Electrodes – Chemistry LibreTexts
Sentek can produce a variety of different types of pH sensitive glass to suit the needs of the application, with a selection of relatively low resistance glass for quick response times or high resistance glass for extremely hostile environments. The temperature of a sample can not only greatly change the resistance of the glass membrane but can also change the activity of the ions in the solution, this is why adding temperature compensation into probes is becoming increasingly popular. We have a selection of temperature compensation selections for all available pH meters.
It’s impossible to explore the anatomy of pH electrodes without considering the external shell. Most electrodes have a glass external body. Glass has very good chemical resistance even for most acids and bases. Glass also allows the user to have a clear view of the inside of their electrode, very useful when the electrode is refillable or to see any contamination. The obvious draw back being is that glass is brittle, so whilst useful in some applications, such as the lab, it may not be well suited to more rugged work in the field. Electrode bodies can be made from durable types of plastic and have even been made into specially machined metal bodies for maximum protection.
Sentek offers a vast range of standard pH electrodes, from the P11 Protected Bulb, to the P11/DJ/NaF for Non-Aqueous Samples. Here at Sentek we can also offer the option to design and build electrodes to your exact specifications. Your unique applications may require specialised solutions, and we have the expertise to tailor our products to your precise requirements.
We understand that selecting the right sensor for your application can be complex, so our dedicated team are ready to assist with any questions you may have.
Get in touch today to discuss your requirements.
Copyright 2024 Sentek Limited. All Rights Reserved.
Marketing by Unity Online